One of my fondest memories of my chiropractic education occurred following a particular lecture on the Philosophy of Chiropractic. I remember leaving the classroom full of resolve and enthusiasm, believing that all I needed to get sick people well was the chiropractic philosophy and a system of specific spinal adjustments. I expressed this idea to a classmate who asked what I would do for a patient who was iron deficient. He asked, “Are you going to put a rusty nail on your pisiform contact and drive the iron into them?”
His question may well have changed my life, because I began to wonder if iron deficiency produced specific subluxation patterns. If so, where and how?
It took me years to determine the answer, and its pursuit led me down a remarkable path of discovery and appreciation for the concept of universal intelligence, not as a philosophical ideal but as an absolute reality—a reality that can be measured and examined and is consequently scientifically sound and legally defensible.
I need no equipment or fancy gadgets to make my determination. All I need is a patient evidencing the symptoms that may suggest iron deficiency, such as fatigue, pallor, and exertional dyspnea. I need my basic science and chiropractic education, and I especially need my hands with which to palpate for both somato-visceral and viscero-somatic involvement.
Iron does not produce subluxation patterns, but the body’s organs do when they are unable to properly digest, absorb, transport and utilize iron. In other words, once we know how the body metabolizes iron, it is quite simple to identify the source of the problem in either the stomach, intestine, spleen, or liver. Obviously, we must begin with a case history with particular emphasis on dietary selections. Innate intelligence is basic science.
Anemia
Anemia is defined by Dorland’s Illustrated Medical Dictionary, 27th edition, as either:
· A deficiency of circulating red blood cells;
· A deficiency of hemoglobin contained within the red blood cells; or,
· A deficiency of the number of packed red blood cells within 100 ml. of blood (hematocrit).
Anemia may be identified by a related nutritional deficiency. These include iron, folate, and vitamin B-12. In this article, we will deal exclusively with iron deficiency, although other factors such as vitamin B-6, vitamin C, and copper may be involved.
Iron Deficiency Anemia
Iron deficiency is the most common cause of anemia, and it is estimated to affect 10-to-20% of the population. The most common causes are:
1. Increased iron requirements for growth that occur not only during infancy and puberty, but during pregnancy and lactation as well.
2. Low dietary intake of iron-containing foods. Often these foods are also high in protein, such as liver, tofu, beans and lentils, some cheeses, and molasses.
3. Obviously, unidentified chronic blood loss can account for iron deficiency. This is particularly true in the gastrointestinal tract. Ulcerations and malignancy can be especially insidious.
4. A less commonly recognized form of anemia occurs when there is a deficient release of iron into the plasma from iron stores. This occurs in chronic inflammation and is not considered to be a true iron deficiency. Nevertheless, it has considerable importance within your practice.
Traditional Recognition of Iron Deficiency
Fatigue is universally considered to be the earliest sign of iron deficiency. However, fatigue has never been correlated with decreased hemoglobin levels. Fatigue, weakness, and anorexia are much more likely to be caused by the depletion of iron-containing enzymes. A small amount of total body iron is incorporated into tissue enzymes. In a dietary deficiency, the level of these tissue enzymes drops BEFORE the hemoglobin level drops.
It takes some time before the clinical manifestations of iron deficiency become evident in the epithelial tissues:
1. Finger nails become thin and flat. Eventually they will become spoon-shaped (koilonychia).
2. The papillae of the tongue will atrophy and will be followed by increased redness and burning. In severe cases, the tongue takes on a smooth and waxy appearance.
3. Inflamed cracks will appear at the corner of the mouth (angular stomatitis).
4. The conjunctiva of the eyes may also appear to pale.
Palpation Findings in Iron Deficiency
Palpation, when properly appreciated, is an incredibly accurate diagnostic science. I don’t mean affixing a medical diagnosis so the indicated drug(s) can be prescribed; I mean ascertaining those very early signs of deviation from normal function that can be appreciated long before evidence of disease can be found. I have written previously that palpatory examinations need not, and should not, be limited to musculoskeletal therapeutics. Nor should they be undervalued; they are much too valuable for recognizing early signs of dysfunction for that.
For example, one very reliable and early sign of impending iron deficiency is muscle contraction of the transverse abdominal muscle on the left side of the abdomen. This occurs immediately below the rib cage and immediately in front of the anterior border of the lumbo-dorsal fascia. This taut and tender muscle contraction (not spasm) has visceral connections with the spleen and the spinal musculature between T6 and T8. While it may be true that spinal subluxation(s) within these segments are responsible, the abdominal finding is more likely found when the spleen cannot furnish adequate red and white blood cells or platelets to maintain normal body function. It is then incumbent upon the practitioner to ascertain the cause, and that can often be surmised by signs and symptoms before a complete blood count would reflect significant changes!
Let’s take a look at how the body metabolizes iron, and then correlate that with findings that appear when normal physiological function is impaired.
Iron Assimilation
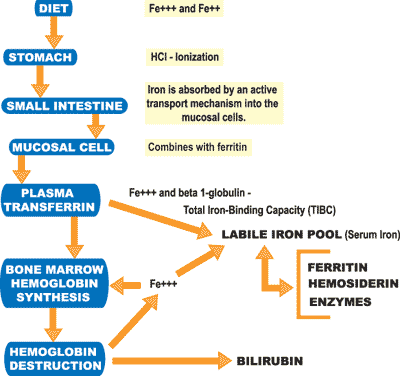
 The daily requirement of iron is about 1 mg in adult males and may be as high as 3-to-4 mg in pregnant women or young menstruating girls. A matching amount should be absorbed from an average dietary intake of 10-to-20 mg per day. On average, everyone loses about 1-to-1.5 mg of iron per day in the feces and urine. Growth, menstruation, and pregnancy obviously increase the amount to be replaced. The average red blood cell lives about 120 days. Therefore, about 1/120th of the total number of red blood cells must be replaced each day. When the old cell breaks, its hemoglobin is recycled into new cells because it would be impossible to replace that much from daily dietary sources.
The daily requirement of iron is about 1 mg in adult males and may be as high as 3-to-4 mg in pregnant women or young menstruating girls. A matching amount should be absorbed from an average dietary intake of 10-to-20 mg per day. On average, everyone loses about 1-to-1.5 mg of iron per day in the feces and urine. Growth, menstruation, and pregnancy obviously increase the amount to be replaced. The average red blood cell lives about 120 days. Therefore, about 1/120th of the total number of red blood cells must be replaced each day. When the old cell breaks, its hemoglobin is recycled into new cells because it would be impossible to replace that much from daily dietary sources.
Dietary iron occurs in food primarily in its oxidized (Fe+++), or ferric, form. A few foods contain it in a reduced (Fe++), or ferrous, form. Animal foods contain iron as hemoglobin (combined with protein).
Digestion
Before dietary iron can be assimilated, it must be removed from its protein connection. Ferric iron must be reduced or put into its ferrous form by stomach acid and is then combined with vitamin C (ascorbate), carbohydrates and amino acids. This process prevents precipitation of the iron as pH rises in the upper intestine. Ascorbate also serves to reduce ferric to ferrous iron, the optimal form for absorption. The heme compound is absorbed and the iron liberated within the mucosal cell.
Iron absorption is decreased whenever the amount of stomach acid is inadequate. This decrease is also found in diseases of the proximal intestinal mucosal surface. Conversely, iron absorption is increased in pancreatic insufficiency by an unknown mechanism.
Any digestive inadequacy will produce palpatory findings in the muscles sharing innervation with the stressed visceral organ. Stomach and biliary problems related to insufficient stomach acid and biliary dysfunction can be palpated under the right costal arch. Pancreatic insufficiency will produce palpatory findings under the left costal arch.
These relationships are very important, because virtually all iron in the body (about one teaspoon) is combined with protein. It is this relationship that allows us to exchange oxygen and carbon dioxide in every cell of the body. A digestive problem interfering with protein will also adversely affect iron metabolism.
Ferritin and Iron Absorption
Once inside the mucosal cell, iron combines with ferritin, a protein. In fact, iron absorption is regulated by available ferritin. When mucosal ferritin is saturated, iron absorption is decreased. On the other hand, when mucosal ferritin is unsaturated, iron is absorbed to fill the gap.
Therefore, blood levels of ferritin can be used to measure iron storage. Low ferritin levels are found in iron deficiency and protein deficiency. High levels of ferritin (iron overload) are considered pathological and may be associated with hemochromatosis, hemosiderosis, megoblastic anemia, alcoholic or inflammatory liver disease, Hodgkin’s disease, breast cancer, and CHRONIC INFLAMMATION (enzyme deficiency). Any chronic condition evidenced by heat (fever), redness, swelling, pain, and aberrant motion can, and usually does, produce iron deficiency anemia!
Serum Iron
When assaying iron levels in the body, we often look only at hemoglobin levels. But we must consider not only ferritin levels, but also serum iron, to understand the entire picture. Low levels of serum iron indicate nutritional iron deficiency. High levels of serum iron are associated with protein deficiency and can be associated with abnormal deposits of iron in the tissues that can damage the liver, heart, lungs, and pituitary. It will cause bronzing of the skin and is referred to as hemochromatosis.
Abnormal deposits of iron in the tissues from multiple transfusions and excess iron administration do not cause damage. This is referred to as hemosiderosis. Hemolytic disease (abnormal destruction of red blood cells) can increase serum iron levels.
Conclusion
There is more to the story of iron, but I have covered the key points for you. Understanding iron metabolism and its relationship to diet, digestion, and inflammation can be very helpful to any clinician. TAC
Howard F. Loomis, Jr., DC, President of Enzyme Formulations, Inc., has an extensive background in enzymes and enzyme supplementation. As president for fifteen years of 21st Century Nutrition (now the Loomis Institute of Enzyme Nutrition), he has forged a remarkable career as an educator, having conducted over 400 seminars to date, in the United States and internationally, on the diagnosis and treatment of food enzyme deficiency syndromes. The Loomis System, Dr. Loomis’ system of evaluation, is recognized as a legitimate and proven method of determining a patient’s nutritional stresses. Call 1-800-662-2630 for more information and a free video.